| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-21 20:27:52 UTC |
|---|
| Update Date | 2014-12-24 20:25:53 UTC |
|---|
| Accession Number | T3D2920 |
|---|
| Identification |
|---|
| Common Name | Flupenthixol |
|---|
| Class | Small Molecule |
|---|
| Description | Flupentixol is an antipsychotic neuroleptic drug. It is a thioxanthene, and therefore closely related to the phenothiazines. Its primary use is as a long acting injection given two or three weekly to people with schizophrenia who have a poor compliance with medication and suffer frequent relapses of illness. It is a D1 and D2 receptor antagonist. It is not approved in the United States. |
|---|
| Compound Type | - Amine
- Antipsychotic Agent
- Dopamine Antagonist
- Drug
- Ether
- Metabolite
- Organic Compound
- Organofluoride
- Synthetic Compound
- Thioxanthene
|
|---|
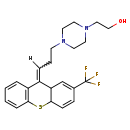
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Depixol | | Fluanxol | | Flupenthixole | | Flupentixol | | Flupentixolo | | Flupentixolum | | Jexit |
|
|---|
| Chemical Formula | C23H27F3N2OS |
|---|
| Average Molecular Mass | 436.533 g/mol |
|---|
| Monoisotopic Mass | 436.180 g/mol |
|---|
| CAS Registry Number | 2709-56-0 |
|---|
| IUPAC Name | 2-(4-{3-[2-(trifluoromethyl)-9,9a-dihydro-4aH-thioxanthen-9-ylidene]propyl}piperazin-1-yl)ethan-1-ol |
|---|
| Traditional Name | flupenthixol |
|---|
| SMILES | [H]\C(CCN1CCN(CCO)CC1)=C1\C2C=C(C=CC2SC2=CC=CC=C12)C(F)(F)F |
|---|
| InChI Identifier | InChI=1/C23H27F3N2OS/c24-23(25,26)17-7-8-22-20(16-17)18(19-4-1-2-6-21(19)30-22)5-3-9-27-10-12-28(13-11-27)14-15-29/h1-2,4-8,16,20,22,29H,3,9-15H2/b18-5- |
|---|
| InChI Key | InChIKey=DTTVNHWDONBIKE-DVZOWYKENA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as thioxanthenes. These are organic polycyclic compounds containing a thioxanthene moiety, which is an aromatic tricycle derived from xanthene by replacing the oxygen atom with a sulfur atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzothiopyrans |
|---|
| Sub Class | 1-benzothiopyrans |
|---|
| Direct Parent | Thioxanthenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Thioxanthene
- Thiochromane
- Aryl thioether
- N-alkylpiperazine
- Alkylarylthioether
- 1,4-diazinane
- Piperazine
- Thiopyran
- Benzenoid
- Tertiary aliphatic amine
- 1,2-aminoalcohol
- Tertiary amine
- Azacycle
- Alkanolamine
- Thioether
- Organic oxygen compound
- Primary alcohol
- Alcohol
- Organooxygen compound
- Organonitrogen compound
- Organofluoride
- Organohalogen compound
- Organic nitrogen compound
- Amine
- Alkyl halide
- Organopnictogen compound
- Alkyl fluoride
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | 0.000346 mg/ml | | LogP | 4.51 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052f-9676500000-6b1399e0dd965860c60c | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0kfx-7343900000-bb450af2259a54640db1 | 2017-10-06 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0201900000-095389998f2b63d484e5 | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0aor-1914300000-8295bd965255669954f6 | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-015i-4901000000-783ed0fccbbc28c48cee | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000900000-9ce18246bedc83edf347 | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-1401900000-aee927a7c2a7b4e811f1 | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9201000000-b5c6ccae989978ac9b0e | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0001900000-bd4e09974af0466a3cbe | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-0016900000-5c47058a37461c2bef3d | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zfs-2239000000-6f83d85a09b5c5a4753e | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000900000-1edd1377901f27944317 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4u-0005900000-4d71ee0a497e6c0b2468 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-0295100000-6274c7e3ec4279e237fd | 2021-10-11 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Fairly slow and incomplete after oral administration |
|---|
| Mechanism of Toxicity | Flupenthixol is a thioxanthene antipsychotic. The mechanism of action of Flupenthixol is not completely understood. Flupenthixol is a powerful antagonist of both D1 and D2 dopamine receptors, and an alpha-adrenergic receptor antagonist. It's antipsychotic activity is thought to be related to blocks postsynaptic dopamine receptors in the CNS. |
|---|
| Metabolism | Mainly hepatic
Half Life: 19 to 39 hours |
|---|
| Toxicity Values | LD50: 300 mk/kg (Oral,Mouse) (1)
LD50: 791 mg/kg (Oral, Rat) (1)
LD50: 87 mk/kg (Intravenous, Mouse) (1)
LD50: 37 mg/kg (Intravenous, Rat) (1) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For use in the treatment of schizophrenia and depression |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00875 |
|---|
| HMDB ID | HMDB15013 |
|---|
| PubChem Compound ID | 25137855 |
|---|
| ChEMBL ID | CHEMBL54661 |
|---|
| ChemSpider ID | Not Available |
|---|
| KEGG ID | C11191 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 5121 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Flupenthixol |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Flupenthixol |
|---|
| References |
|---|
| Synthesis Reference | Smith Kline & French Laboratories; British Patent 925,538; May 8, 1963.
Craig, P.N. and Zirkle, C.L.; U.S. Patent 3,282,930; November 1, 1966; assigned to Smith Kline & French Laboratories. |
|---|
| MSDS | T3D2920.pdf |
|---|
| General References | - Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M: DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008 Jan;36(Database issue):D901-6. Epub 2007 Nov 29. [18048412 ]
- Drugs.com [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|