| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-10-14 21:20:17 UTC |
|---|
| Update Date | 2014-12-24 20:27:01 UTC |
|---|
| Accession Number | T3D4988 |
|---|
| Identification |
|---|
| Common Name | 9-(2-Carboxyphenyl)-3,6-bis(diethylamino)xanthylium(1+) |
|---|
| Class | Small Molecule |
|---|
| Description | 9-(2-Carboxyphenyl)-3,6-bis(diethylamino)xanthylium(1+) is a food dye
9-(2-Carboxyphenyl)-3,6-bis(diethylamino)xanthylium(1+) belongs to the family of Xanthenes. These are polycyclic aromatic compounds containing a xanthene moiety, which consists of two benzene ring joined to each other by a pyran ring. |
|---|
| Compound Type | - Dye
- Food Additive
- Food Toxin
- Metabolite
- Synthetic Compound
|
|---|
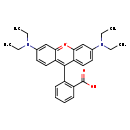
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 81-88-9 (CHLORIDE) | | 9-(2-Carboxyphenyl)-3,6-bis(diethylamino)-Xanthylium | | 9-(2-Carboxyphenyl)-3,6-bis(diethylamino)xanthylium | | 9-(2-Carboxyphenyl)-3,6-bis(diethylamino)xanthylium(1+), 9CI | | Basazol Red 71P | | Basic rose red | | Basic Violet 10 | | C.I. 45170 | | C.I. Basic Violet 10 | | C.I. Food Red 15 | | C.I. Solvent Red 49 | | Calcozine red BX | | Calcozine rhodamine BXP | | Cerise Toner X 1127 | | D And C Red 19 | | Eriosin rhodamine b | | Food Red 15 | | N-[9-(2-Carboxyphenyl)-6-(diethylamino)-3H-xanthen-3-ylidene]-N-ethylethanaminium(1+), 9CI | | Pilot 578 | | Rhodamine | | Rhodamine 610 | | Rhodamine B | | Rhodamine b cation | | Rhodamine b monocation | | Rhodamine B(1+) | | Rhodamine lake red b | | Tetraethylrhodamine |
|
|---|
| Chemical Formula | C28H31N2O3 |
|---|
| Average Molecular Mass | 443.557 g/mol |
|---|
| Monoisotopic Mass | 443.233 g/mol |
|---|
| CAS Registry Number | 14899-08-2 |
|---|
| IUPAC Name | 9-(2-carboxyphenyl)-6-(diethylamino)-N,N-diethyl-3H-xanthen-3-iminium |
|---|
| Traditional Name | rhodamine B(1+) |
|---|
| SMILES | CCN(CC)C1=CC2=[O+]C3=C(C=CC(=C3)N(CC)CC)C(C3=CC=CC=C3C(O)=O)=C2C=C1 |
|---|
| InChI Identifier | InChI=1S/C28H30N2O3/c1-5-29(6-2)19-13-15-23-25(17-19)33-26-18-20(30(7-3)8-4)14-16-24(26)27(23)21-11-9-10-12-22(21)28(31)32/h9-18H,5-8H2,1-4H3/p+1 |
|---|
| InChI Key | InChIKey=CVAVMIODJQHEEH-UHFFFAOYSA-O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as xanthenes. These are polycyclic aromatic compounds containing a xanthene moiety, which consists of two benzene rings joined to each other by a pyran ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzopyrans |
|---|
| Sub Class | 1-benzopyrans |
|---|
| Direct Parent | Xanthenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Xanthene
- Benzoic acid or derivatives
- Benzoic acid
- Benzoyl
- Tertiary aliphatic/aromatic amine
- Dialkylarylamine
- Monocyclic benzene moiety
- Benzenoid
- Heteroaromatic compound
- Amino acid or derivatives
- Amino acid
- Tertiary amine
- Oxacycle
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic nitrogen compound
- Amine
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organonitrogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic cation
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Endogenous |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | The solubility of Rhodamine B in water is ~15 g/L.[8] However, the solubility in acetic acid solution (30 vol.%) is ~400 g/L. (Wikipedia) | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03fr-0201900000-6ddae7ec527f63d82258 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-004s-2000910000-f514b6402bfea591b5c4 | 2017-10-06 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0000900000-2fdde36060a9fe440262 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kg-0000900000-9c87be1c845dc583a654 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ba-6113900000-d8bc00aae3733b299974 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0000900000-410660bd6e9676b52dea | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-1000900000-eb15465a3f6f966326f3 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006x-9002400000-16b1da2c93179f10c79b | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kg-0003900000-580a90bf751eaa24e325 | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0007-0006900000-7a20165467799e0cafd7 | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ta-0009200000-b43e0a84f39a69884713 | 2021-09-23 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 3, not classifiable as to its carcinogenicity to humans. (2) |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB31786 |
|---|
| PubChem Compound ID | Not Available |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | Not Available |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | - Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC.
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|