| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-09-11 05:23:23 UTC |
|---|
| Update Date | 2014-12-24 20:27:00 UTC |
|---|
| Accession Number | T3D4942 |
|---|
| Identification |
|---|
| Common Name | Fenpropimorph |
|---|
| Class | Small Molecule |
|---|
| Description | Fenpropimorph is an Agricultural fungicide used against powdery mildews on sugar beet, beans and leeks
Fenpropimorph has been shown to exhibit anti-fungal function (1). |
|---|
| Compound Type | - Amine
- Ether
- Food Toxin
- Fungicide
- Metabolite
- Organic Compound
- Pesticide
- Synthetic Compound
|
|---|
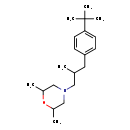
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 4-[3-(4-Tert-Butylphenyl)-2-methylpropyl]-2,6-dimethylmorpholine | | 4-[3-[4-(1,1-Dimethylethyl)phenyl]-2-methylpropyl]-2,6-dimethylmorpholine, 9CI | | Corbel | | Fenpropemorph | | Fenpropimorphe | | Funbas | | Mildofix | | Mistral | | Mistral t | | Power task |
|
|---|
| Chemical Formula | C20H33NO |
|---|
| Average Molecular Mass | 303.482 g/mol |
|---|
| Monoisotopic Mass | 303.256 g/mol |
|---|
| CAS Registry Number | 67306-03-0 and 67564-91-4 |
|---|
| IUPAC Name | 4-{2-[(4-tert-butylphenyl)methyl]propyl}-2,6-dimethylmorpholine |
|---|
| Traditional Name | corbel |
|---|
| SMILES | CC(CN1CC(C)OC(C)C1)CC1=CC=C(C=C1)C(C)(C)C |
|---|
| InChI Identifier | InChI=1/C20H33NO/c1-15(12-21-13-16(2)22-17(3)14-21)11-18-7-9-19(10-8-18)20(4,5)6/h7-10,15-17H,11-14H2,1-6H3 |
|---|
| InChI Key | InChIKey=RYAUSSKQMZRMAI-UHFFFAOYNA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylpropanes. These are organic compounds containing a phenylpropane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Phenylpropanes |
|---|
| Direct Parent | Phenylpropanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylpropane
- Aralkylamine
- Morpholine
- Oxazinane
- Tertiary amine
- Tertiary aliphatic amine
- Organoheterocyclic compound
- Ether
- Dialkyl ether
- Azacycle
- Oxacycle
- Hydrocarbon derivative
- Amine
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | < 25 °C | | Boiling Point | Not Available | | Solubility | 1 mg/mL | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-004i-2900000000-7f6db950cf70b2276bc8 | 2014-06-16 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0553-6980000000-2b1e972ae02c02b91fa8 | 2017-09-01 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-0f6t-1903000000-01b715c061ada400c79a | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-0002-1900000000-39af7489cf29ff80ad70 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-0udi-0509000000-969288f065ea3db41ac6 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-000t-0900000000-9099f4920ef471897461 | 2017-09-14 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0619000000-166a2e3c2bdf20ad866f | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0911000000-6d9a212283d4df4ce244 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dr-2900000000-18bbb94efbe5b52edb03 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0109000000-79342889054f3f41f773 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0529000000-b622d2ef84634000f9f6 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9110000000-87184ac21af54d90f81d | 2016-08-03 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB37270 |
|---|
| PubChem Compound ID | 91695 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 82798 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 50148 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D4942.pdf |
|---|
| General References | - Stolz J, Sauer N: The fenpropimorph resistance gene FEN2 from Saccharomyces cerevisiae encodes a plasma membrane H+-pantothenate symporter. J Biol Chem. 1999 Jun 25;274(26):18747-52. [10373490 ]
- Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC.
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|