| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-09-11 05:19:44 UTC |
|---|
| Update Date | 2014-12-24 20:26:58 UTC |
|---|
| Accession Number | T3D4862 |
|---|
| Identification |
|---|
| Common Name | Disodium 6-hydroxy-5-[(4-sulfophenyl)azo]-2-naphthalenesulfonate |
|---|
| Class | Small Molecule |
|---|
| Description | Disodium 6-hydroxy-5-[(4-sulfophenyl)azo]-2-naphthalenesulfonate is a food dye
Disodium 6-hydroxy-5-[(4-sulfophenyl)azo]-2-naphthalenesulfonate belongs to the family of Naphthalenes. These are compounds containing a naphthalene moiety, which consists of two fused benzene rings. |
|---|
| Compound Type | - Dye
- Food Additive
- Food Toxin
- Household Toxin
- Metabolite
- Organic Compound
- Synthetic Compound
|
|---|
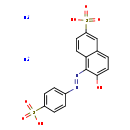
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 1351 Yellow, disodium salt | | 1899 Yellow, disodium salt | | Acid Food Yellow 3, disodium salt | | Alabaster No. 3, disodium salt | | C.I. Food Yellow 3, disodium salt | | Canacert sunset yellow FCF, disodium salt | | Certicol sunset yellow CFS, disodium salt | | Disodium 6-hydroxy-5-[(4-sulfophenyl)azo]-2-naphthalenesulfonic acid | | Disodium 6-hydroxy-5-[(4-sulphophenyl)azo]-2-naphthalenesulfonate | | Disodium 6-hydroxy-5-[(4-sulphophenyl)azo]-2-naphthalenesulfonic acid | | FD and C Yellow No. 6, disodium salt | | Food Yellow No. 5, disodium salt | | HD sunset yellow FCF, disodium salt | | Hexacol sunset yellow FCF, disodium salt | | Maple sunset yellow FCF, disodium salt | | Sun yellow, disodium salt | | Sunset Yellow 6, disodium salt | | Twilight yellow, disodium salt | | Yellow No. 6, disodium salt |
|
|---|
| Chemical Formula | C16H12N2Na2O7S2 |
|---|
| Average Molecular Mass | 454.384 g/mol |
|---|
| Monoisotopic Mass | 453.987 g/mol |
|---|
| CAS Registry Number | 2783-94-0 |
|---|
| IUPAC Name | disodium 6-hydroxy-5-[(E)-2-(4-sulfophenyl)diazen-1-yl]naphthalene-2-sulfonic acid |
|---|
| Traditional Name | disodium 6-hydroxy-5-[(E)-2-(4-sulfophenyl)diazen-1-yl]naphthalene-2-sulfonic acid |
|---|
| SMILES | [Na+].[Na+].OC1=C(\N=N\C2=CC=C(C=C2)S(O)(=O)=O)C2=CC=C(C=C2C=C1)S(O)(=O)=O |
|---|
| InChI Identifier | InChI=1S/C16H12N2O7S2.2Na/c19-15-8-1-10-9-13(27(23,24)25)6-7-14(10)16(15)18-17-11-2-4-12(5-3-11)26(20,21)22;;/h1-9,19H,(H,20,21,22)(H,23,24,25);;/q;2*+1/b18-17+;; |
|---|
| InChI Key | InChIKey=OIQPTROHQCGFEF-QIKYXUGXSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 2-naphthalene sulfonates. These are organic aromatic compounds that contain a naphthalene moiety that carries a sulfonic acid group at the 2-position. Naphthalene is a bicyclic compound that is made up of two fused benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Naphthalenes |
|---|
| Sub Class | Naphthalene sulfonic acids and derivatives |
|---|
| Direct Parent | 2-naphthalene sulfonates |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2-naphthalene sulfonate
- 2-naphthalene sulfonic acid or derivatives
- 2-naphthol
- Benzenesulfonate
- Arylsulfonic acid or derivatives
- Benzenesulfonyl group
- 1-sulfo,2-unsubstituted aromatic compound
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Organosulfonic acid
- Sulfonyl
- Azo compound
- Organic alkali metal salt
- Organic nitrogen compound
- Organic oxygen compound
- Organic salt
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Organic cation
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 390 °C | | Boiling Point | Not Available | | Solubility | 190 mg/mL at 25 °C | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | Not Available |
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 3, not classifiable as to its carcinogenicity to humans. (2) |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB30683 |
|---|
| PubChem Compound ID | Not Available |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | Not Available |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | - Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC.
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|