| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-09-11 05:17:21 UTC |
|---|
| Update Date | 2014-12-24 20:26:57 UTC |
|---|
| Accession Number | T3D4804 |
|---|
| Identification |
|---|
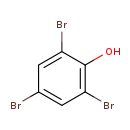
| Common Name | 2,4,6-Tribromophenol |
|---|
| Class | Small Molecule |
|---|
| Description | 2,4,6-Tribromophenol is found in crustaceans. 2,4,6-Tribromophenol is isolated from molluscs and crustaceans. 2,4,6-Tribromophenol is a flavour component of seafood, imparts an intense shrimp-like flavour. 2,4,6-Tribromophenol belongs to the family of Bromobenzenes. These are organic compounds containing a chlorine atom attached to a benzene ring. |
|---|
| Compound Type | - Animal Toxin
- Bromide Compound
- Food Toxin
- Marine Toxin
- Metabolite
- Natural Compound
- Organic Compound
- Organobromide
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 2,4,6-Tribromo-3-methylphenol | | 2,4,6-Tribromo-m-cresol | | 2,4,6-Tribromo-Phenol | | 2,4,6-Tribromophenol, bismuth (3+) salt | | 5175-83-7 (Bismuth(3+) salt) | | Bismuth tribromophenate | | Bromkal Pur 3 | | Bromol | | C6H3Br3O | | Flammex 3BP | | Great lakes PH-73 | | Micatex | | TBP | | Tribromophenol | | Xeroform |
|
|---|
| Chemical Formula | C6H3Br3O |
|---|
| Average Molecular Mass | 330.799 g/mol |
|---|
| Monoisotopic Mass | 327.773 g/mol |
|---|
| CAS Registry Number | 118-79-6 |
|---|
| IUPAC Name | 2,4,6-tribromophenol |
|---|
| Traditional Name | 2,4,6-tribromophenol |
|---|
| SMILES | OC1=C(Br)C=C(Br)C=C1Br |
|---|
| InChI Identifier | InChI=1S/C6H3Br3O/c7-3-1-4(8)6(10)5(9)2-3/h1-2,10H |
|---|
| InChI Key | InChIKey=BSWWXRFVMJHFBN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as p-bromophenols. These are bromophenols carrying a iodine at the C4 position of the benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenols |
|---|
| Sub Class | Halophenols |
|---|
| Direct Parent | P-bromophenols |
|---|
| Alternative Parents | |
|---|
| Substituents | - 4-bromophenol
- 2-bromophenol
- Halobenzene
- Bromobenzene
- Monocyclic benzene moiety
- Aryl halide
- Aryl bromide
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organobromide
- Organohalogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Endogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 95.5°C | | Boiling Point | 286°C | | Solubility | 70 mg/L (at 15°C) | | LogP | 4.13 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0059-3329000000-8507237252f6c68a3e56 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0059-3329000000-8507237252f6c68a3e56 | 2018-05-18 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0059-0119000000-1a9e2f72fef4a0ac8111 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00di-7109000000-97e2aa9549b71aa7b589 | 2017-10-06 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-01q9-9318000000-c2e1e322c2756de014c5 | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, CDCl3, experimental) | Not Available | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 15.09 MHz, CDCl3, experimental) | Not Available | 2014-09-23 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | This is an endogenously produced metabolite found in the human body. It is used in metabolic reactions, catabolic reactions or waste generation. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB02417 |
|---|
| HMDB ID | HMDB29642 |
|---|
| PubChem Compound ID | 1483 |
|---|
| ChEMBL ID | CHEMBL220087 |
|---|
| ChemSpider ID | 1438 |
|---|
| KEGG ID | C14454 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 47696 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | C004554 |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | TBP |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D4804.pdf |
|---|
| General References | - Oliveira AS, Silva VM, Veloso MC, Santos GV, Andrade JB: Bromophenol concentrations in fish from Salvador, BA, Brazil. An Acad Bras Cienc. 2009 Jun;81(2):165-72. [19488620 ]
- Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC.
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|