| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-08-29 05:00:13 UTC |
|---|
| Update Date | 2014-12-24 20:26:37 UTC |
|---|
| Accession Number | T3D4061 |
|---|
| Identification |
|---|
| Common Name | Veratridine |
|---|
| Class | Small Molecule |
|---|
| Description | Veratridine is a steroid-derived alkaloid from plants in the Liliaceae family that functions as a neurotoxin by activating sodium ion channels. It is primarily obtained from the herb Veratrum and sabadilla seeds. It binds to intramembrane receptor site 2 and increases intracellular Ca2+ concentration. It acts by preferentially binding to activated Na+ channels causing persistent activation that leads to increased nerve excitability. |
|---|
| Compound Type | - Amine
- Ester
- Ether
- Natural Compound
- Organic Compound
- Plant Toxin
|
|---|
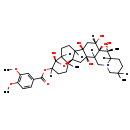
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | (3•_,4alpha-,16•_)-4,12,14,16,17,20-hexahydroxy-4,9-epoxycevan-3-yl 3,4-dimethoxybenzoate |
|
|---|
| Chemical Formula | C36H51NO11 |
|---|
| Average Molecular Mass | 673.790 g/mol |
|---|
| Monoisotopic Mass | 673.346 g/mol |
|---|
| CAS Registry Number | 71-62-5 |
|---|
| IUPAC Name | (1R,10R,11S,12S,14R,23S,25R)-1,10,11,12,14,23-hexahydroxy-6,10,19-trimethyl-24-oxa-4-azaheptacyclo[12.12.0.0²,¹¹.0⁴,⁹.0¹⁵,²⁵.0¹⁸,²³.0¹⁹,²⁵]hexacosan-22-yl 3,4-dimethoxybenzoate |
|---|
| Traditional Name | (1R,10R,11S,12S,14R,23S,25R)-1,10,11,12,14,23-hexahydroxy-6,10,19-trimethyl-24-oxa-4-azaheptacyclo[12.12.0.0²,¹¹.0⁴,⁹.0¹⁵,²⁵.0¹⁸,²³.0¹⁹,²⁵]hexacosan-22-yl 3,4-dimethoxybenzoate |
|---|
| SMILES | [H]C12CCC3([H])[C@]4(O)C[C@]([H])(O)[C@@]5(O)C([H])(CN6CC([H])(C)CCC6([H])[C@@]5(C)O)[C@]4(O)C[C@@]33O[C@]1(O)C([H])(CCC23C)OC(=O)C1=CC(OC)=C(OC)C=C1 |
|---|
| InChI Identifier | InChI=1S/C36H51NO11/c1-19-6-11-26-31(3,40)35(43)25(17-37(26)16-19)33(42)18-34-24(32(33,41)15-27(35)38)10-9-23-30(34,2)13-12-28(36(23,44)48-34)47-29(39)20-7-8-21(45-4)22(14-20)46-5/h7-8,14,19,23-28,38,40-44H,6,9-13,15-18H2,1-5H3/t19?,23?,24?,25?,26?,27-,28?,30?,31+,32+,33+,34+,35-,36-/m0/s1 |
|---|
| InChI Key | InChIKey=FVECELJHCSPHKY-UIHGVQCZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cerveratrum-type alkaloids. These are steroidal alkaloids containing the cevane (23-methyl-4- azahexacyclo[12.11.0.0^{2,11}.0^{4,9}.0^{15,24}.0^{18,23}]pentacosane) moiety, which is a six ring system. Cerveratrum alkaloids have 7-9 oxygen atoms and occur as free alkamines or esters of simple aliphatic or aromatic acids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Steroidal alkaloids |
|---|
| Direct Parent | Cerveratrum-type alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cerveratrum-type alkaloid
- Azasteroid
- M-methoxybenzoic acid or derivatives
- P-methoxybenzoic acid or derivatives
- Benzoate ester
- Quinolizidine
- Dimethoxybenzene
- O-dimethoxybenzene
- Benzoic acid or derivatives
- Alkaloid or derivatives
- Anisole
- Phenoxy compound
- Benzoyl
- Phenol ether
- Methoxybenzene
- Oxepane
- Alkyl aryl ether
- Benzenoid
- Piperidine
- Monocyclic benzene moiety
- Tetrahydrofuran
- Tertiary alcohol
- Cyclic alcohol
- Tertiary amine
- Secondary alcohol
- Amino acid or derivatives
- Tertiary aliphatic amine
- 1,2-aminoalcohol
- Carboxylic acid ester
- Hemiacetal
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Polyol
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Ether
- Alcohol
- Organooxygen compound
- Organic nitrogen compound
- Organic oxygen compound
- Organonitrogen compound
- Amine
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cell surface
- Cytoplasm
- Cytosol
- Endoplasmic reticulum
- Endosome
- Extracellular
- Extracellular matrix
- Lysosome
- Membrane Fraction
- Microtubule
- Mitochondrial Matrix
- Mitochondrial Membrane
- Mitochondrion
- Nerve Fiber
- Perinuclear region
- Plasma Membrane
- Sarcoplasmic Reticulum
- Secretory Granule
- Secretory vesicle
- Synaptic Vesicle
- Tubulin
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | | Name | SMPDB Link | KEGG Link |
|---|
| Apoptosis | Not Available | map04210 | | Oxidative phosphorylation | Not Available | map00190 | | Insulin secretion | Not Available | map04911 | | Vascular smooth muscle contraction | Not Available | map04270 | | Anticonvulsants | Not Available | Not Available | | Cell cycle | Not Available | map04110 | | Pancreatic secretion | Not Available | map04972 | | Phenothiazines | Not Available | Not Available | | Long-term potentiation | Not Available | map04720 | | Antiarrhythmic Drugs | Not Available | Not Available | | Fatty acid Metabolism | SMP00051 | map00071 | | Endocytosis | Not Available | map04144 | | Eicosanoids | Not Available | Not Available |
|
|---|
| Applications | Not Available |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05fr-0000209000-3237a2bdd832d2444200 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ab9-0101109000-c5201e08ab9ba9b0ef48 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dm-5600619000-a48eade657a0b301a9ea | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0200009000-f87b37a1ff8901bf1366 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0089-0900116000-37885115e65f1290eac8 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-0900000000-3056855f4ba192868e8d | 2016-08-03 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Veratridine is a steroid-derived alkaloid from plants in the Liliaceae family that functions as a neurotoxin by abolishing inactivation of sodium ion channels. It binds to intramembrane receptor site 2 and increases intracellular Ca2+ concentration. It acts by preferentially binding to activated Na+ channels causing persistent activation that leads to increased nerve excitability. (Wikipedia) The activation of the action potential Na+ ionophore by veratridine is time- and concentrationdependent and completely reversible. (1) |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Veratridine is a steroid-derived alkaloid from plants in the Liliaceae family that functions as a neurotoxin by activating sodium ion channels. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 441081 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 5290571 |
|---|
| KEGG ID | C06544 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D4061.pdf |
|---|
| General References | - Catterall WA: Activation of the action potential Na+ ionophore of cultured neuroblastoma cells by veratridine and batrachotoxin. J Biol Chem. 1975 Jun 10;250(11):4053-9. [1168643 ]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|