| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-11-13 22:46:58 UTC |
|---|
| Update Date | 2014-12-24 20:26:13 UTC |
|---|
| Accession Number | T3D3606 |
|---|
| Identification |
|---|
| Common Name | Ochratoxin B |
|---|
| Class | Small Molecule |
|---|
| Description | Ochratoxin B is a metabolite of Aspergillus ochraceus
Ochratoxin B belongs to the family of Ochratoxins and related substances. These are compounds containing the ochratoxin skeleton, which is structurally characterized by the presence of a 3-phenylpropanoic acid N-linked to a 8-hydroxy-3-methyl-1-oxo-3,4-dihydro-1H-2-benzopyran-7-carboxamide moiety[1]. (Reference: [1] http://www.inchem.org/documents/ehc/ehc/ehc105.htm). |
|---|
| Compound Type | - Amide
- Amine
- Ester
- Ether
- Food Toxin
- Fungal Toxin
- Metabolite
- Mycotoxin
- Natural Compound
- Organic Compound
|
|---|
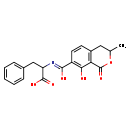
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 2-[(8-Hydroxy-3-methyl-1-oxoisochroman-7-carbonyl)amino]-3-phenylpropionic acid | | N-[(8-Hydroxy-3-methyl-1-oxo-3,4-dihydro-1H-isochromen-7-yl)carbonyl]phenylalanine |
|
|---|
| Chemical Formula | C20H19NO6 |
|---|
| Average Molecular Mass | 369.368 g/mol |
|---|
| Monoisotopic Mass | 369.121 g/mol |
|---|
| CAS Registry Number | 4825-86-9 |
|---|
| IUPAC Name | 2-{[hydroxy(8-hydroxy-3-methyl-1-oxo-3,4-dihydro-1H-2-benzopyran-7-yl)methylidene]amino}-3-phenylpropanoic acid |
|---|
| Traditional Name | 2-{[hydroxy(8-hydroxy-3-methyl-1-oxo-3,4-dihydro-2-benzopyran-7-yl)methylidene]amino}-3-phenylpropanoic acid |
|---|
| SMILES | CC1CC2=C(C(=O)O1)C(O)=C(C=C2)C(O)=NC(CC1=CC=CC=C1)C(O)=O |
|---|
| InChI Identifier | InChI=1/C20H19NO6/c1-11-9-13-7-8-14(17(22)16(13)20(26)27-11)18(23)21-15(19(24)25)10-12-5-3-2-4-6-12/h2-8,11,15,22H,9-10H2,1H3,(H,21,23)(H,24,25) |
|---|
| InChI Key | InChIKey=DAEYIVCTQUFNTM-UHFFFAOYNA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as ochratoxins and related substances. These are a group of chemically related metabolites containing a 3,4-dihydro-3-methylisocoumarin moiety linked through a carboxyl group to L-beta-phenylalanine by a secondary amine bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Ochratoxins and related substances |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Ochratoxins and related substances |
|---|
| Alternative Parents | |
|---|
| Substituents | - Ochratoxin-skeleton
- Phenylalanine or derivatives
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- 3-phenylpropanoic-acid
- Alpha-amino acid or derivatives
- Amphetamine or derivatives
- Salicylic acid or derivatives
- 2-benzopyran
- Isochromane
- Benzopyran
- 1-hydroxy-4-unsubstituted benzenoid
- Benzenoid
- Dicarboxylic acid or derivatives
- Monocyclic benzene moiety
- Vinylogous acid
- Lactone
- Secondary carboxylic acid amide
- Carboxylic acid ester
- Carboxamide group
- Oxacycle
- Carboxylic acid derivative
- Organoheterocyclic compound
- Carboxylic acid
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | Crystals from methanol. (4) |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 221°C | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9188000000-465239f3bd78eb30bd63 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-00di-6012890000-f11d1dffcd9b915bacf9 | 2017-10-06 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-2219000000-50c4b901f9dd1f436db4 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05fu-2597000000-1f43b9bf5e9f0cb80117 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9400000000-0ea895a91c60938f8217 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00xr-0029000000-8b9a1400d7de1b8460f5 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0100-2669000000-b399dc5e8324895bef28 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-6930000000-95579f686cf729c65a22 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0gi0-0829000000-8c84a51e9e11593d1cd5 | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0gi4-3849000000-6305fce231bb3e0b71bc | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ugl-3940000000-d7838720f0f845c19ee7 | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0009000000-c8e6150e81e908b0971e | 2021-09-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0l6r-0946000000-c51245f8d3561a8a0bd0 | 2021-09-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fml-1920000000-ad9d01b16eda880f5d9b | 2021-09-24 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral, dermal, inhalation, and parenteral (contaminated drugs). (3) |
|---|
| Mechanism of Toxicity | Ochratoxin B is thought to inhibit the accumulation of cartilage proteoglycans and general protein synthesis in a concentration-related manner. (2) |
|---|
| Metabolism | Ochratoxin B is metabolized to 4-hydroxyochratoxin B. (1) |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Human exposure occurs mainly through consumption of improperly stored food products, particularly contaminated grain and pork products, as well as coffee, wine grapes and dried grapes. (9) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Ochratoxin exposure has been associated with acute tubular necrosis and Balkan endemic nephropathy. Ochratoxin A has been shown to be nephrotoxic; might delay sexual maturation. (5) |
|---|
| Symptoms | Might cause respiratory irritation. (5) |
|---|
| Treatment | Care is symptomatic and supportive. (5) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB29401 |
|---|
| PubChem Compound ID | 609663 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 529965 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D3606.pdf |
|---|
| General References | - Stormer FC, Kolsaker P, Holm H, Rogstad S, Elling F: Metabolism of ochratoxin B and its possible effects upon the metabolism and toxicity of ochratoxin A in rats. Appl Environ Microbiol. 1985 May;49(5):1108-12. [4004232 ]
- Wiger R, Stormer FC: Effects of ochratoxins A and B on prechondrogenic mesenchymal cells from chick embryo limb buds. Toxicol Lett. 1990 Dec;54(2-3):129-34. [2260111 ]
- Peraica M, Domijan AM: Contamination of food with mycotoxins and human health. Arh Hig Rada Toksikol. 2001 Mar;52(1):23-35. [11370295 ]
- Rothenberg SP, Marcoullis GP, Schwarz S, Lader E: Measurement of cyanocobalamin in serum by a specific radioimmunoassay. J Lab Clin Med. 1984 Jun;103(6):959-72. [6726060 ]
- Grond S, Sablotzki A: Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879-923. [15509185 ]
- Rumack BH POISINDEX(R) Information System Micromedex, Inc., Englewood, CO, 2010; CCIS Volume 143, edition expires Feb, 2010. Hall AH & Rumack BH (Eds): TOMES(R) Information System Micromedex, Inc., Englewood, CO, 2010; CCIS Volume 143, edition expires Feb, 2010.
- O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1209
- Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC.
- Wikipedia. Ochratoxin A. Last Updated 26 February 2010. [Link]
- Wikipedia. Ochratoxin. Last Updated 29 March 2010. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|