| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-21 20:28:12 UTC |
|---|
| Update Date | 2014-12-24 20:25:54 UTC |
|---|
| Accession Number | T3D2963 |
|---|
| Identification |
|---|
| Common Name | Ethinamate |
|---|
| Class | Small Molecule |
|---|
| Description | Ethinamate is a short-acting sedative-hypnotic medication used to treat insomnia. Regular use leads to tolerance, and it is usually not effective for more than 7 days. Structurally, it does not resemble the barbituates, but it shares many effects with this class of drugs; the depressant effects of ethinamate are, however, generally milder than those of most barbiturates. |
|---|
| Compound Type | - Amine
- Carbamate
- Drug
- Ester
- Ether
- Hypnotic and Sedative
- Metabolite
- Organic Compound
- Synthetic Compound
|
|---|
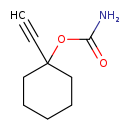
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 1-Ethynylcyclohexanol carbamate | | Aethinyl-cyclohexyl-carbamat | | Ethinamat | | Ethinamatum | | Ethinamic acid | | Ethinimate | | Etinamate | | Etinamato | | USAF EL-42 | | Valamin | | Valmid |
|
|---|
| Chemical Formula | C9H13NO2 |
|---|
| Average Molecular Mass | 167.205 g/mol |
|---|
| Monoisotopic Mass | 167.095 g/mol |
|---|
| CAS Registry Number | 126-52-3 |
|---|
| IUPAC Name | 1-ethynylcyclohexyl carbamate |
|---|
| Traditional Name | ethinamate |
|---|
| SMILES | OC(=N)OC1(CCCCC1)C#C |
|---|
| InChI Identifier | InChI=1S/C9H13NO2/c1-2-9(12-8(10)11)6-4-3-5-7-9/h1H,3-7H2,(H2,10,11) |
|---|
| InChI Key | InChIKey=GXRZIMHKGDIBEW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as ynones. These are organic compounds containing the ynone functional group, an alpha,beta unsaturated ketone group with the general structure RC#C-C(=O)R' (R' not H). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | Ynones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Ynone
- Carbamic acid ester
- Carbonic acid derivative
- Acetylide
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organonitrogen compound
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 94-96°C | | Boiling Point | 120°C at 3.00E+00 mm Hg | | Solubility | 2500 mg/L (at 25°C) | | LogP | 1.2 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-054o-9000000000-115ca538c5db7623cd36 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - CI-B (Non-derivatized) | splash10-004i-0900000000-3530aac44af3672b4ea8 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-054o-9000000000-115ca538c5db7623cd36 | 2018-05-18 | View Spectrum | | GC-MS | GC-MS Spectrum - CI-B (Non-derivatized) | splash10-004i-0900000000-3530aac44af3672b4ea8 | 2018-05-18 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052f-9400000000-b2399bc8d370f3c24953 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-016r-1900000000-eae16bf8bad39a8aa79b | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056r-4900000000-5017ae578b8cb02cd66d | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ktf-9100000000-cd1eedbdd6d6fcbfa22e | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-006x-9800000000-e408920834450f49d3fe | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006x-9500000000-877c16eefea2f5a15d9b | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9300000000-b9a9228e0ee207499b99 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-2900000000-10552185ed182d4ba1e0 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9700000000-cd8eef43ebf47b3d3b7b | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-9100000000-9313282cbda800790a68 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0avl-2900000000-d0731a549bbc943d745e | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9500000000-35acbd59dd232e271712 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-ff48c45d4a47d2073ba7 | 2021-10-11 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0arv-9100000000-713c62aa52e71fc3560a | 2014-09-20 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Rapidly absorbed following oral administration. |
|---|
| Mechanism of Toxicity | The mechanism of action is not known. However, studies have shown that ethinamate inhibits carbonic anhydrases I and II (J Biol Chem. 1992 Dec 15;267(35):25044-50). This inhibition by ethinamate is not sufficiently strong, however, to implicate carbonic anhydrases I and II in the mechanism of action. |
|---|
| Metabolism | Hepatic.
Half Life: 2.5 hours |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Used for the short-term treatment of insomnia, however, it generally has been replaced by other sedative-hypnotic agents. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | They cause slurred speech, disorientation and "drunken" behavior. They are physically and psychologically addictive. |
|---|
| Symptoms | Symptoms of overdose include shortness of breath or slow or troubled breathing, slow heartbeat, severe weakness, chronic confusion, slurred speech, and staggering. |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01031 |
|---|
| HMDB ID | HMDB15165 |
|---|
| PubChem Compound ID | 3284 |
|---|
| ChEMBL ID | CHEMBL1576 |
|---|
| ChemSpider ID | 3169 |
|---|
| KEGG ID | C07832 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 4884 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Ethinamate |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Ethinamate |
|---|
| References |
|---|
| Synthesis Reference | Junkmann, K. and Pfeiffer, H.; US. Patent 2,816,910; December 17, 1957; assigned to
Schering AG, Germany. |
|---|
| MSDS | Not Available |
|---|
| General References | - Drugs.com [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|