| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-05 03:12:29 UTC |
|---|
| Update Date | 2014-12-24 20:25:42 UTC |
|---|
| Accession Number | T3D2562 |
|---|
| Identification |
|---|
| Common Name | Gamma-Butyrolactone |
|---|
| Class | Small Molecule |
|---|
| Description | One of the furans with a carbonyl thereby forming a cyclic lactone. It is an endogenous compound made from gamma-aminobutyrate and is the precursor of gamma-hydroxybutyrate. It is also used as a pharmacological agent and solvent. |
|---|
| Compound Type | - Ester
- Ether
- Food Toxin
- Household Toxin
- Indicator and Reagent
- Industrial/Workplace Toxin
- Metabolite
- Organic Compound
- Solvent
- Synthetic Compound
|
|---|
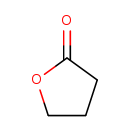
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 1,4-Butanolide | | 1,4-Lactone | | 1-Oxacyclopentan-2-one | | 2,3,4,5-Tetrahydro-2-furanone | | 2-Oxolanone | | 2-Oxotetrahydrofuran | | 4,5-Dihydro-2(3H)-furanone | | 4-Butanolide | | 4-Deoxytetronate | | 4-Deoxytetronic acid | | 4-Hydroxy-Butanoate | | 4-Hydroxy-Butanoic acid | | 4-Hydroxy-Butanoic acid g-lactone | | 4-Hydroxybutanoate | | 4-Hydroxybutanoic acid | | 4-Hydroxybutanoic acid lactone | | 4-Hydroxybutyric acid lactone | | Butyric acid lactone | | Butyrolactone | | Dihydro-2(3H)-furanone | | dihydrofuran-2(3h)-one | | g-Butalactone | | g-Butyrolactone | | g-Butyryllactone | | g-Hydroxybutyric acid lactone | | gamma-Butalactone | | gamma-Butyrolactone | | gamma-Butyryllactone | | gamma-Hydroxybutyric acid lactone | | GBL | | Paint Clean G | | Tetrahydro-2-furanone | | γ-Butyrolactone |
|
|---|
| Chemical Formula | C4H6O2 |
|---|
| Average Molecular Mass | 86.089 g/mol |
|---|
| Monoisotopic Mass | 86.037 g/mol |
|---|
| CAS Registry Number | 96-48-0 |
|---|
| IUPAC Name | oxolan-2-one |
|---|
| Traditional Name | butyrolactone |
|---|
| SMILES | O=C1CCCO1 |
|---|
| InChI Identifier | InChI=1S/C4H6O2/c5-4-2-1-3-6-4/h1-3H2 |
|---|
| InChI Key | InChIKey=YEJRWHAVMIAJKC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as gamma butyrolactones. Gamma butyrolactones are compounds containing a gamma butyrolactone moiety, which consists of an aliphatic five-member ring with four carbon atoms, one oxygen atom, and bears a ketone group on the carbon adjacent to the oxygen atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Lactones |
|---|
| Sub Class | Gamma butyrolactones |
|---|
| Direct Parent | Gamma butyrolactones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Gamma butyrolactone
- Tetrahydrofuran
- Carboxylic acid ester
- Oxacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Endogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Colorless oily liquid (18). |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | -45°C | | Boiling Point | Not Available | | Solubility | 1000.0 mg/mL | | LogP | -0.64 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-004l-9000000000-500445dc73cb69119e6b | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0006-9000000000-c2f6d51e7b2f9ce1e9f1 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-002f-9000000000-d3df175d3315ed446e14 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-004l-9000000000-500445dc73cb69119e6b | 2018-05-18 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0006-9000000000-c2f6d51e7b2f9ce1e9f1 | 2018-05-18 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-002f-9000000000-d3df175d3315ed446e14 | 2018-05-18 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-002f-9000000000-bd4b413f20907d13a107 | 2016-09-22 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-000i-9000000000-4a6de93563809aa51a33 | 2012-07-24 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-000i-9000000000-4a6de93563809aa51a33 | 2012-07-24 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-000i-9000000000-b5163f6eb1e8004e61a9 | 2012-07-24 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI RMU-7M) , Positive | splash10-004l-9000000000-1066d349a6023c66dd64 | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - EI-B (JEOL JMS-D-3000) , Positive | splash10-0006-9000000000-c2f6d51e7b2f9ce1e9f1 | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI M-80B) , Positive | splash10-002f-9000000000-d3df175d3315ed446e14 | 2012-08-31 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-9000000000-fb7c9ada825a0c62979f | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000l-9000000000-95fda8a898d02f7e71ff | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000l-9000000000-c8282365f175b61ca8c0 | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-9000000000-ec20127c74818b1f634d | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052r-9000000000-86d21b481322417edf81 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-4f454124d6cece18a212 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-9000000000-507998514018a44c4cf0 | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000l-9000000000-d4ec4947321dfb11fab4 | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-b2a1436205f5365513b0 | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-9000000000-709821dd006aa045c8b0 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9000000000-ffe2d9473d85a58305c1 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-a4219a074939d10a0f5f | 2021-09-22 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-004l-9000000000-2fc3f4165b5808b41e93 | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | Not Available | 2012-12-04 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, CDCl3, experimental) | Not Available | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 25.16 MHz, CDCl3, experimental) | Not Available | 2014-09-23 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, CDCl3, experimental) | Not Available | 2021-10-10 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | 2012-12-05 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Inhalation (2) ; oral (2) ; injection (2) ; dermal (2). |
|---|
| Mechanism of Toxicity | Gamma-butyrolactone is rapidly converted to gamma-hydroxybutyrate. This may account for the subsequent central nervous system depressant. Gamma-butyrolactone is an anesthetic that causes a selective increase in brain dopamine by antagonizing transmitter release from nerve terminal. It is also an endogenous brain metabolite that may be derived from glutamate through gamma-aminobutyrate. GBL binds to the picrotoxin receptor (3, 1). |
|---|
| Metabolism | Gamma-Butyrolactone undergoes rapid and quantitative conversion by lactonases, yielding gamma-hydroxybutyric acid (4). |
|---|
| Toxicity Values | LD50: 17.2 mL/kg (Oral, Rat) (581) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 3, not classifiable as to its carcinogenicity to humans. (17) |
|---|
| Uses/Sources | Common solvent and reagent in chemistry and is used as an aroma compound, as a stain remover, as a superglue remover, as a paint stripper, and as a solvent in some wet aluminium electrolytic capacitors (18). |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | CNS depression; amnesia and hypotonia. Rarely, hypertension, orthostatic hypotension, apnea, dyskinesias, dystonias, and hypomania occur. |
|---|
| Symptoms | Headache, confusion, ataxia, urinary incontinence or urgency, difficulty breathing, bradycardia, uncontrollable shaking, hallucinations, seizure-like activity (uncontrollable or unusual movements), and acidosis. Hypothermia has been reported in adults and children following exposure (13).
|
|---|
| Treatment | In case of oral exposure, administer a benzodiazepine IV. There is no antidote. Treatment is symptomatic and supportive. (13)
|
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB04699 |
|---|
| HMDB ID | HMDB00549 |
|---|
| PubChem Compound ID | 7302 |
|---|
| ChEMBL ID | CHEMBL95681 |
|---|
| ChemSpider ID | 7029 |
|---|
| KEGG ID | C01770 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 42639 |
|---|
| BioCyc ID | CPD-10135 |
|---|
| CTD ID | D015107 |
|---|
| Stitch ID | Butyrolactone, gamma- |
|---|
| PDB ID | GBL |
|---|
| ACToR ID | 227 |
|---|
| Wikipedia Link | 4-Hydroxybutyric acid lactone |
|---|
| References |
|---|
| Synthesis Reference | Fabio Giannessi, Maria Ornella Tinti, Francesco De Angelis, “Process for producing®-3-hydroxy-4-butyrolactone useful for preparing®-carnitine.” U.S. Patent US6127552, issued February, 1998. |
|---|
| MSDS | Link |
|---|
| General References | - Holland KD, Yoon KW, Ferrendelli JA, Covey DF, Rothman SM: Gamma-butyrolactone antagonism of the picrotoxin receptor: comparison of a pure antagonist and a mixed antagonist/inverse agonist. Mol Pharmacol. 1991 Jan;39(1):79-84. [1846222 ]
- McMahon LR, Cunningham KA: Role of 5-HT(2a) and 5-HT(2B/2C) receptors in the behavioral interactions between serotonin and catecholamine reuptake inhibitors. Neuropsychopharmacology. 2001 Mar;24(3):319-29. [11166521 ]
- Zhang M, Hu P, Krois CR, Kane MA, Napoli JL: Altered vitamin A homeostasis and increased size and adiposity in the rdh1-null mouse. FASEB J. 2007 Sep;21(11):2886-96. Epub 2007 Apr 13. [17435174 ]
- Winter JC, Fiorella DJ, Helsley SE, Rabin RA: Partial generalization of (-)DOM to fluvoxamine in the rat: implications for SSRI-induced mania and psychosis. Int J Neuropsychopharmacol. 1999 Sep;2(3):165-172. [11281985 ]
- Fukui Y, Matsusima E, Muramoto K, Nagai N, Ohama K, Yamashita K: Validation of a simple gas chromatographic-mass spectrometric method for the determination of gamma-butyrolactone in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2003 Feb 25;785(1):73-80. [12535840 ]
- Thupari JN, Landree LE, Ronnett GV, Kuhajda FP: C75 increases peripheral energy utilization and fatty acid oxidation in diet-induced obesity. Proc Natl Acad Sci U S A. 2002 Jul 9;99(14):9498-502. Epub 2002 Jun 11. [12060712 ]
- Wood M, Laloup M, Samyn N, Morris MR, de Bruijn EA, Maes RA, Young MS, Maes V, De Boeck G: Simultaneous analysis of gamma-hydroxybutyric acid and its precursors in urine using liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2004 Nov 12;1056(1-2):83-90. [15595536 ]
- Quintanilla RA, Orellana DI, Gonzalez-Billault C, Maccioni RB: Interleukin-6 induces Alzheimer-type phosphorylation of tau protein by deregulating the cdk5/p35 pathway. Exp Cell Res. 2004 Apr 15;295(1):245-57. [15051507 ]
- Knust U, Hull WE, Spiegelhalder B, Bartsch H, Strowitzki T, Owen RW: Analysis of enterolignan glucuronides in serum and urine by HPLC-ESI-MS. Food Chem Toxicol. 2006 Jul;44(7):1038-49. Epub 2006 Feb 20. [16488523 ]
- Selmaoui B, Aymard N, Lambrozo J, Touitou Y: Evaluation of the nocturnal levels of urinary biogenic amines in men exposed overnight to 50-Hz magnetic field. Life Sci. 2003 Oct 31;73(24):3073-82. [14550848 ]
- Oshima I, Saito S, Shiota K, Miyake A, Oka Y, Nakayama R: Kinetic study on disappearance of gamma-butyrolactone-gamma-carbonyl-L-histidyl-L-prolinamide (DN-1417) from plasma using a radioimmunoassay for DN-1417 isobutylamide. J Pharmacobiodyn. 1983 Mar;6(3):202-8. [6410041 ]
- Yeatman DT, Reid K: A study of urinary endogenous gamma-hydroxybutyrate (GHB) levels. J Anal Toxicol. 2003 Jan-Feb;27(1):40-2. [12587682 ]
- Rumack BH (2009). POISINDEX(R) Information System. Englewood, CO: Micromedex, Inc. CCIS Volume 141, edition expires Aug, 2009.
- Ellenhorn MJ, Schonwald S, Ordog G, Wasserberger J (1997). Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins.
- NIOSH (1990). NOES: National Occupational Exposure Survey conducted from 1981-1983. Estimated numbers of employees potentially exposed to specific agents by 2-digit standard industrial classification (SIC). National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication.
- BG Chemie (2000). Toxicological Evaluation No. 7: gamma-Butyrolactone (96-48-0).
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
- Wikipedia. Gamma-Butyrolactone. Last Updated 1 August 2009. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|